UNIFIED COGNITIVE RESPONSE:
BUNTANETAP SHOWS THE STRONGEST BENEFIT IN AMYLOID/TAU-POSITIVE PATIENTS WITH MMSE >20




ALZHEIMER'S STUDIES
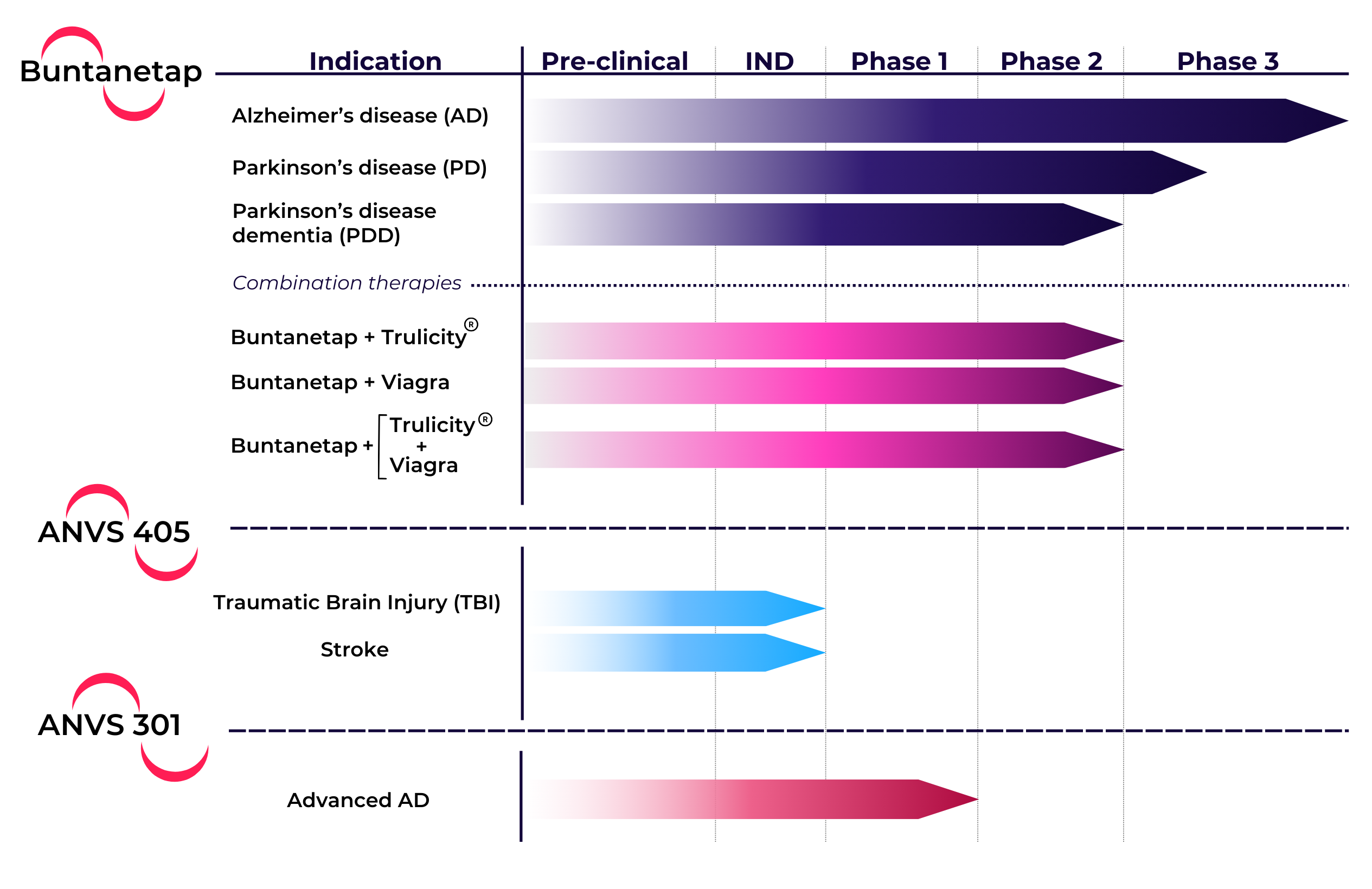
Phase 3 Study - recruiting
A Double-blind Dual Study Assessing Safety and Efficacy of Buntanetap in Participants with Early AD
Study goal: To determine whether a daily dose (30mg) of buntanetap is effective in treating early AD
Duration: 6-month (symptomatic) & 18-month (disease-modifying)
Key inclusion criteria: Adults aged 55-85, plasma pTau217-positive, MMSE 21-28
Estimated enrollment: 760 patients
Clinical sites: 83
More information can be found in Patient Portal or at clinicaltrials.gov.
Phase 2/3 Study
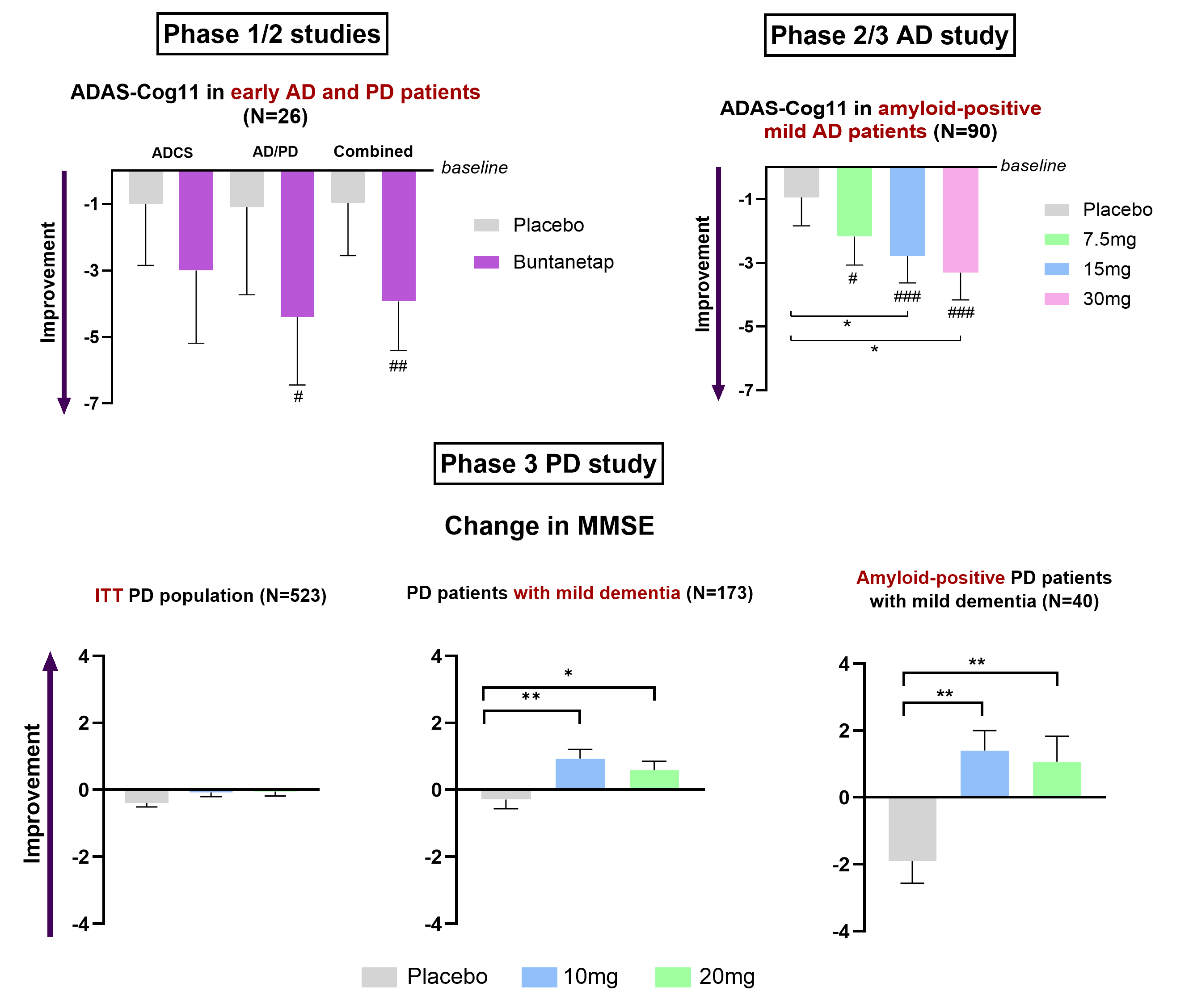
The study was completed with 345 patients, of which 90 were identified as having mild AD (MMSE 21-24). These 90 AD patients showed dose-dependent, statistically significant improvements from placebo in the two higher concentrations (15mg, p=0.042; 30mg, p=0.015) and all doses showed significant improvement over baseline (7.5mg, p= 0.013; 15mg p=0.001; 30mg p<0.001).
The data further showed that APOE4 carriers respond well to the drug improving by 3 - 4 points in ADAS-Cog11, similar to the response seen in non-carriers.
Improved cognition in mild AD patients (a) and, specifically, in APOE4 carriers (b)

Neurotoxic proteins
For additional information see – News Releases
PARKINSON'S STUDIES
Phase 3 Study
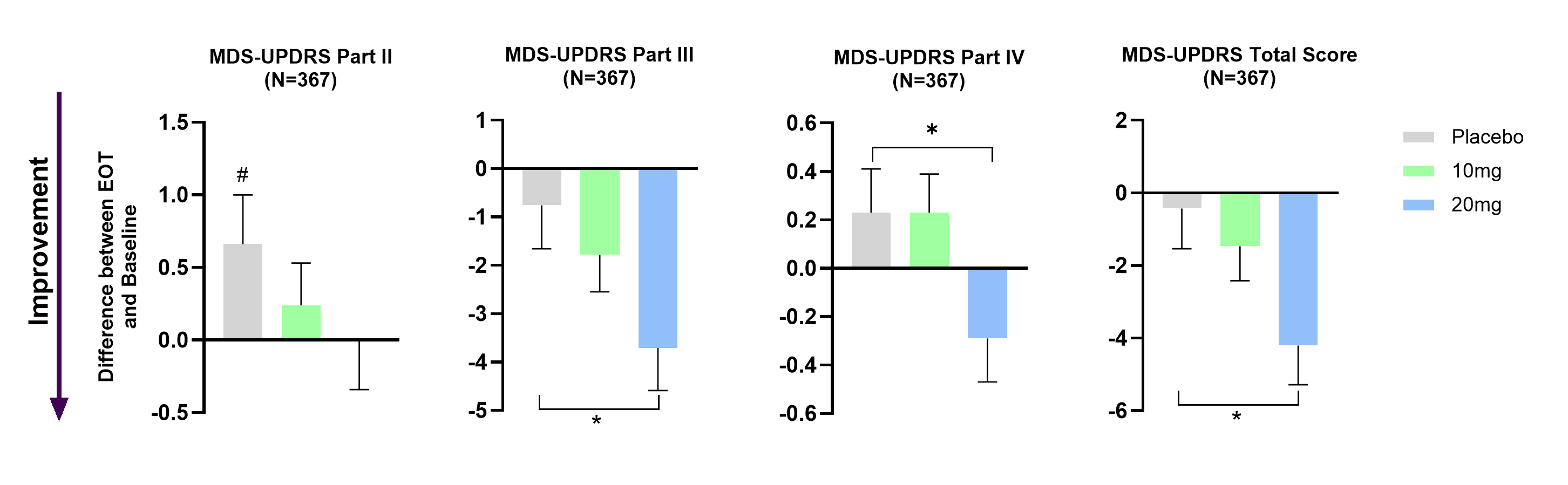
A total of 471 patients completed our Phase 3 study in early Parkinson’s disease.
Buntanetap demonstrated clinically meaningful improvements across all key MDS-UPDRS measures (Parts II, III, IV, and Total scores) both in the Per-Protocol-Population and in patients with Postural Instability and Gait Difficulties (PIGD).
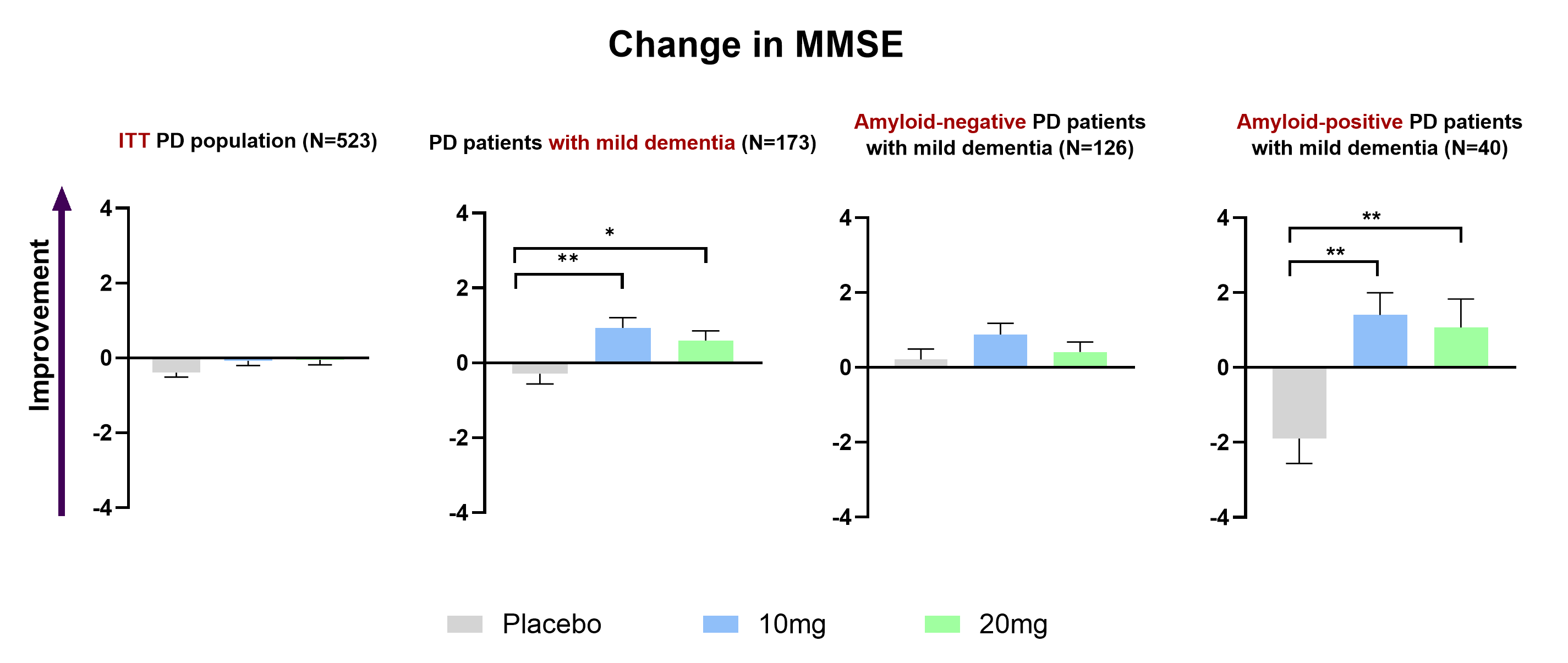
Importantly, buntanetap halted cognitive decline.
While patients on placebo experienced worsening of cognition (MMSE) over six months, those treated with buntanetap showed no decline.
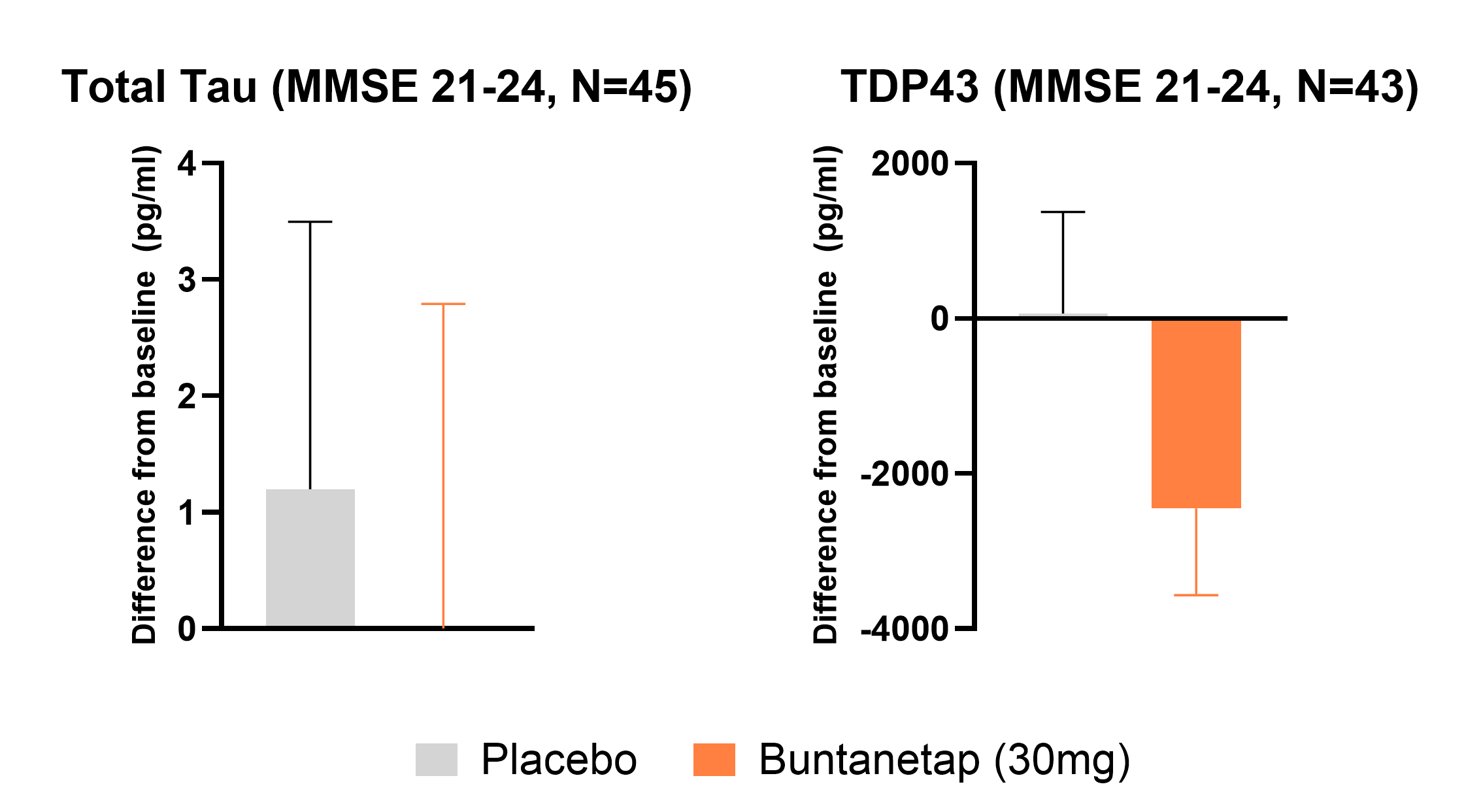
The strongest cognitive benefits were seen in patients at higher risk for decline.
In individuals with mild dementia (MMSE >20) who were also positive for amyloid and tau, buntanetap produced the most pronounced cognitive improvements.
Per-Protocol-Population
Phase 2a Studies
Two Phase 2a studies in AD patients demonstrated an improvement in cognitive functions over baseline after 20-28 days of treatment with buntanetap.
In the Phase 2a Discover study, 60mg of buntanetap given for 20 days showed a strong trend in improving ADAS-Cog11 score, while in Phase 2a AD/PD study, 80mg buntanetap given over 25 days showed clinically significant improvement (p=0.04) in ADAS-Cog11 by 4.4 points from baseline and 3.3 points over placebo (MMSE 18-28).
Improved cognition (a) and speed/accuracy (b) in Alzheimer's

Data from 14 AD patients showed that ADAS-Cog11 improved by 4.4 points from baseline in the buntanetap-treated group, a statistically significant improvement of 30% (p<0.05). At 25 days, the treated group was also 3.3 points better than the placebo. The WAIS coding, which measured speed in movement and thinking, showed a statistically significant 23% improvement in AD patients treated with buntanetap compared to baseline.
Improved movement (a) and speed/accuracy (b) in Parkinson's

All buntanetap treatment groups combined showed statistically significant improvement in the MDS-UPDRS Part III and WAIS coding test. In this study, patients receiving 10mg and 20mg of buntanetap were the best performing groups. (* p<0.05; ** p<0.01; *** p<0.001).
Publication: Fang et al., 2022

.svg)

.svg)
.svg)
.svg)
